Legal Update
Dec 3, 2025
California Proposition 65: What In‑House Counsel Need to Know About the New Short-Term Warning Regulations
Executive Summary
California updated the Proposition 65 safe harbor warning rules, effective January 1, 2025, with a three‑year transition through January 1, 2028. The short‑form warning now must name at least one specific chemical per applicable endpoint (cancer and/or reproductive toxicity) and may use the signal words “WARNING,” “CA WARNING,” or “CALIFORNIA WARNING.” The rules clarify online warnings and allow short‑form warnings for food; tailored warnings were added for passenger/off‑highway motor vehicle parts and recreational marine vessel parts.
Why it matters: The updated short‑form requires chemical‑specific identification and consistent execution across labels, product pages, and catalogs. There is unlimited sell‑through for inventory manufactured and labeled before Jan 1, 2028, but new production after that date must use the revised short‑form. Internet retailers have a 60‑day window to update online warnings after manufacturers change label content during the transition period.
Enforcement reality: Private enforcement continues to grow (5,000+ notices in 2024), frequently targeting metals, phthalates, PFAS (e.g., PFOA), and bisphenols (e.g., BPA/BPS). Two recent federal injunctions narrowed enforcement for dietary acrylamide and titanium dioxide in cosmetics/personal care, but overall risk remains high across many categories.
Action items for compliance: (1) Conduct inventory & risk triage; (2) Evaluate testing & documentation; (3) label & web warning updates; (4) Alignment of suppliers & retailers; (5) governance & training.
At a Glance: What Changed (and When)
The California Office of Environmental Health Hazard Assessment (“OEHHA”) sought to increase transparency and reduce over‑warning by requiring businesses to identify at least one chemical in the short‑form warning, while offering California‑specific signal words. The agency concluded consumers need more information than the previous short‑form provided; the new approach balances specificity with practical label constraints.
Effective January 1stof this year, the revised warnings were required for new products, with the transition period lasting through January 1, 2028. Any inventory manufactured and labeled before January 1, 2028, can keep the prior warnings but new production after Jan 1, 2028 must use the revised short‑form warning content.
As noted above, the changes are as follows:
- Core short‑form changes:
(1) Name at least one listed chemical per applicable endpoint (cancer/reproductive).
(2) Optional signal words: “WARNING,” “CA WARNING,” “CALIFORNIA WARNING.”
(3) Short‑form may be used on any label size if ≥ 6‑point font and conspicuous.
(4) Short‑form may be used for food; tailored warnings added for passenger/off‑highway motor vehicle parts and recreational marine vessel parts.
- Internet & catalog: Warnings must be prominent on product display pages or clearly marked hyperlinks using the words “WARNING,” “CA WARNING,” or “CALIFORNIA WARNING: before checkout. Retailers have a 60‑day window during the transition period to update online warnings after manufacturer notice. If the product label uses the short‑form, the website may use the same content.
Here are some examples of the new short-form warnings:
- Carcinogen‑only:
“Cancer risk from exposure to [name of chemical]. See www.P65Warnings.ca.gov.”
or
“Can expose you to [name of chemical], a carcinogen. See www.P65Warnings.ca.gov.”
- Reproductive toxicity‑only:
“Reproductive Harm/Reproductive Toxicant risk from exposure to [name of chemical]. See www.P65Warnings.ca.gov.”
or
“Can expose you to [name of chemical], a reproductive toxicant. See www.P65Warnings.ca.gov.”
- Both carcinogen and reproductive toxicity:
“Risk of cancer from exposure to [name of chemical] and reproductive harm from exposure to [name of chemical]. See www.P65Warnings.ca.gov.”
or
“Can expose you to [name of chemical], a carcinogen, and [name of chemical], a reproductive toxicant. See www.P65Warnings.ca.gov.”
Enforcement Landscape: Trends to Expect
Private enforcement activity continued to rise in 2024, targeting food, personal care, and hardgoods; metals and phthalates remain common, and PFAS (notably PFOA) notices increased substantially. 2024 in‑court settlements topped $76 million, underscoring litigation stakes. Two federal injunctions currently limit enforcement for dietary acrylamide and titanium dioxide in cosmetics/personal care; monitor appellate and regulatory developments.
PFAS Compliance Under California Law and Proposition 65
PFAS (per‑ and poly‑fluoroalkyl substances) are a top compliance and enforcement priority in California. Beyond Prop 65, product‑category statutes restrict PFAS in textiles, juvenile products, cookware/food packaging, and menstrual products, with DTSC registration and enforcement schedules ramping over the coming years.
Key statutes and timelines: AB 1817 (textiles), AB 652 (juvenile products), and AB 1200 (cookware/food packaging) establish PFAS restrictions and disclosures. AB 347 (2024) gives DTSC enforcement authority and requires manufacturer registration by July 1, 2029, with enforcement beginning after July 1, 2030. AB 2515 (2024) prohibits menstrual products with regulated PFAS—intentional addition prohibited beginning Jan 1, 2025; concentration‑based limit to be set by DTSC beginning Jan 1, 2027; registration required by July 1, 2029.
Prop 65 interplay: PFOA/PFOS are frequently cited in notices for apparel, accessories, and coated materials. If a Prop 65 warning is required (or chosen), the new short‑form must name the specific PFAS (e.g., PFOA). Mirror label content online with prominent product page warnings or clearly marked “WARNING,” “CA WARNING,” “CALIFORNIA WARNING.” hyperlinks before checkout.
Practical Implications for In‑House Counsel
- Safe harbor vs. bespoke warnings: safe harbor provides presumption of compliance but now demands specific chemical identification.
- Strengthen chemical mapping/testing: use BOM reviews, supplier attestations, and targeted lab work to identify the named chemical per endpoint.
- Update labels and digital content together: synchronize signal words, chemical names, icon/URL conventions, ≥ 6‑pt font, and conspicuous placement across packaging and product pages.
- Segment by category: maintain food vs. non‑food templates and leverage tailored warnings for auto/marine parts.
- Coordinate upstream/downstream: contract for supplier disclosures and retailer update obligations; align ecommerce workflows for timely online warnings.
- Monitor listings and litigation: track acrylamide and TiO2 injunctions, bisphenol listing activity, and evolving PFAS guidance.
Common Pitfalls to Avoid
As with any other regulatory changes, some best practices can be implemented to avoid liability exposure. First, avoid using legacy short‑form content (without chemical names) on products manufactured on or after January 1, 2028. For internet sales, careful review is required to ensure that warnings are listed prior to check out and that they are prominent without burying WARNING links on product pages. Further, to comply with the new regulations, there cannot be a mismatch between label and website content when the short‑form is used.
Be sure to investigate your particular products – there are now food‑specific conventions (URL, symbol practice) for safe harbor alignment.
Action Plan: The Next 12 Months
Here are some recommendations for ensuring compliance with the regulations, if you have not taken these steps already:
- Inventory review and risk triage for California‑sold SKUs.
- Testing and exposure assessment; document rationales and safe harbor evaluations.
- Label template redesign for revised short‑form (chemical names, signal words, icon/URL, ≥ 6‑pt font).
- Ecommerce and catalog synchronization with standardized WARNING links and placement; set 60‑day reviews for updates.
- Supplier and retailer contracts: disclosures, testing protocols, change‑notice obligations, and indemnities.
- Governance and training for packaging, ecommerce, and customer‑facing teams.
- Monitoring for OEHHA/DTSC notices and any 60‑day notices received.
Conclusion
Short-form warnings are not required but they provide a presumption of compliance with Prop. 65 requirements. Bespoke warnings must still be “clear and reasonable” and defensible. While you do not need to list multiple chemicals if more than one is present, you do need to list at least one chemical per endpoint. If one chemical is listed for both endpoints, you may name that single chemical but be sure to include both the reproductive and carcinogen risks.
For multi‑SKU products for online sales, you will need to provide a warning on each product display page or a clearly marked hyperlink to the warning before checkout; mirror label content online.
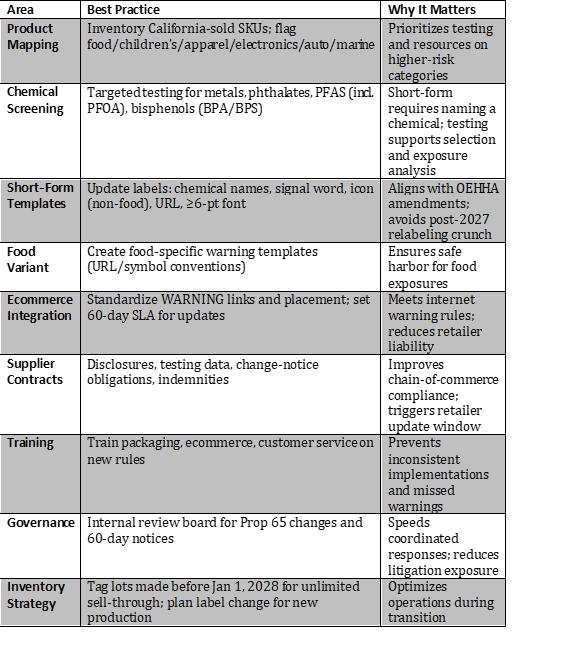
Compliance Checklist: Best Practices Timeline

Seyfarth Shaw LLP provides this information as a service to clients and other friends for educational purposes only. It should not be construed or relied on as legal advice or to create a lawyer-client relationship. Readers should not act upon this information without seeking advice from their professional advisers.
